Stability
Enhancement of n-Gaas Characteristics by Combined Heating, Cooling Rate And Metalloporphyrin Modification Techniques
Mon, 2014-03-03 12:07 — Iyad SAADEDDINDifferent modification techniques, namely, preheating, controlling the cooling rate and modification with tetra(-4-pyridyl)porphyrinatomanganese(III) have been used to enhance photoelectrochemical characteristics of n-GaAs electrodes in light-to-electricity conversions. Combination of such three techniques together yielded electrodes with better darkcurrent density vs potential plots and photocurrent density vs potential plots. Higher efficiency and stability were also observed for electrodes modified by such combined techniques.

Dissolution Rate and Stability Study of Flavanone Aglycones, Naringenin And Hesperetin, By Drug Delivery Systems Based on Polyvinylpyrrolidone (PVP) Nanodispersions
Mon, 2013-12-30 13:47 — Feras I. QanazeObjective: To study the dissolution behavior, the release mechanism and the stability of nanodispersion system of aglycones with PVP. Methods: The nanodispersion system of polyvinylpyrrolidone (PVP)/naringenin–hesperetin was prepared using the solvent evaporation method. The chemical stability (compatibility) of naringenin and hesperetin in the prepared dispersions was studied under accelerated conditions for 3 months. The evaluation of physical stability was performed by X-ray diffraction analysis (XRD) and by comparing the dissolution profile before and after storage at high temperature and moisture (40ºC, RH 75%). Results: The dissolution rate of naringenin and hesperetin released was dramatically increased in the nanodispersion system of PVP/naringenin–hesperetin (80/20, w/w). The release mechanism of both flavanone aglycones was better described by the diffusion model (Higuchi model). Also it was found that the rate-limiting step that controlled the release of naringenin and hesperetin in the nanodispersion system was dissolution of the carrier (PVP). Conclusions: During accelerated degradation analysis, for 3 months at high temperature and moisture, PVP nanodispersion system showed enhanced chemical compatibility and physical stability. The physical evaluation (obtained from XRD analysis) of PVP/naringenin–hesperetin (80/20, w/w) in the selected storage conditions did not show any crystallization of flavanone aglycones in the PVP nanodispersion system or any change in their release profile.
Metalloporphyrin/Polysiloxane Modified N-Gaas Surfaces: Effect on Photoelectrochemical Efficiency and Surface Stability
Wed, 2013-04-24 11:25 — Samar Al ShakhshirTetra(-4-pyridyl)porphyrinatomanganese(III)sulfate (as an MnIII+MnII ion mixture) was embedded into a polysiloxane polymer matrix and attached to the surfaces of the n-GaAs electrode. The n-GaAs/polymer/MnP system was annealed under nitrogen and used for a photoelectrochemical study in a water/LiClO4/Fe(CN)6 3 −/Fe(CN)6 4− system. The values of short-circuit currents, measured after minutes of illumination, were significantly enhanced by modification. The modified electrode surfaces were more stable to degradation, in the dark and under illumination, than the unmodified ones. Furthermore, the modified electrodes showed higher light-to-electricity conversion efficiency than the unmodified ones. The methodology described here is advantageous in the sense that the semiconductor electrode properties can be enhanced in more than one aspect at the same time.
A validated and Stability Indicating HPLC Method for Analysis of Diminazene Aceturate and Antipyrine Combination in a Ready Injectable Solution
Sun, 2013-03-31 09:59 — Murad N AbualhasanDiminazene aceturate and Antipyrine combination therapy is widely used in veterinary medicine. A simple reverse HPLC method for the analysis of samples of a ready injectable formulation containing a mixture of active ingredients and inactive excipients has been developed. The HPLC analysis was carried out using a reversed phase (RP)-C18 (250 mm×4.0 mm, 5 μm) column. The isocratic mobile phase consisted of a mixture of acetonitrile, methanol, phosphate buffer and hexane sulfonate; the flow rate was 0.6 mL/min and ultraviolet detection was at 291 nm. This method was validated in accordance with FDA and ICH guidelines and showed good linearity, accuracy, precision, selectivity and the system suitability results were within the acceptance criteria. A stability-indicating study was also carried out and indicated that this method could be used for purity and degradation evaluation of these formulations.
Tablet Formulation and Development of a Validated Stability Indicating HPLC Method for Quantification of Valsartan and Hydrochlorthiazide Combination
Tue, 2013-02-12 08:16 — Murad N AbualhasanEnhancement of n-Gaas Characteristics by Combined Heating, Cooling Rate And Metalloporphyrin Modification Techniques
Wed, 2011-03-09 09:08 — Hikmat S. Hilal, D.Sc.Different modification techniques, namely, preheating, controlling the cooling rate and modification with tetra(-4-pyridyl)porphyrinatomanganese(III) have been used to enhance photoelectrochemical characteristics of n-GaAs electrodes in light-to-electricity conversions. Combination of such three techniques together yielded electrodes with better darkcurrent density vs potential plots and photocurrent density vs potential plots. Higher efficiency and stability were also observed for electrodes modified by such combined techniques.
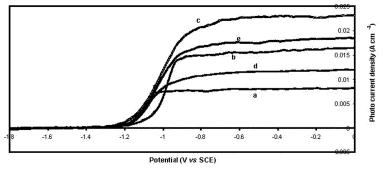
Metalloporphyrin/Polysiloxane Modified N-Gaas Surfaces: Effect on Photoelectrochemical Efficiency and Surface Stability
Wed, 2011-03-09 09:04 — Hikmat S. Hilal, D.Sc.Tetra(-4-pyridyl)porphyrinatomanganese(III)sulfate (as an MnIII+MnII ion mixture) was embedded into a polysiloxane polymer matrix and attached to the surfaces of the n-GaAs electrode. The n-GaAs/polymer/MnP system was annealed under nitrogen and used for a photoelectrochemical study in a water/LiClO4/Fe(CN)6 3 −/Fe(CN)6 4− system. The values of short-circuit currents, measured after minutes of illumination, were significantly enhanced by modification. The modified electrode surfaces were more stable to degradation, in the dark and under illumination, than the unmodified ones. Furthermore, the modified electrodes showed higher light-to-electricity conversion efficiency than the unmodified ones. The methodology described here is advantageous in the sense that the semiconductor electrode properties can be enhanced in more than one aspect at the same time.
Terminal Olefin Isomerization Reactions Catalyzed by Poly(Siloxane)-Supported Ru3(CO)12 : The Effect of The Support on the Catalyst Selectivity, Activity and Stability
Wed, 2011-03-09 08:56 — Hikmat S. Hilal, D.Sc.Dodecacarbonyltriruthenium(0), Ru3(CO)12, 1, has been chemically anchored to the aminated polysiloxane surface, 2. The resulting supported ruthenium complex, 3, was evaluated as catalyst for the olefin isomerization reactions. Contrary to its homogeneous catalyst counterpart, 1, the supported catalyst 3 showed exceptionally high selectivity towards 1-octene isomerization, and trans-2-octene was the sole product of the reaction mixture. The olefin isomerization reaction was markedly activated by the presence of the tertiary silane (EtO)3SiH. No hydrosilylation reaction products were detected. Preliminary kinetic study indicated catalysis by lower nuclearity catalytic species, where the cluster fragments during the reaction process. The effects of different reaction parameters on the rate of the reaction have been investigated.
Effect of Cooling Rate of Pre-Annealed Cds Thin Film Electrodes Prepared by Chemical Bath Deposition: Enhancement of Photoelectrochemical Characteristics
Tue, 2011-03-08 13:20 — Iyad SAADEDDINThin films of CdS, deposited by chemical bath deposition (CBD) onto films of fluorine-doped tin oxide/glass (glass/FTO) substrates were prepared and investigated for photoelectrochemical conversion (PEC) of light into electricity. Knowing the hazardous nature of CdS, the focal theme of this work was to modify the electrodes by simple economic ways to maximize their conversion efficiency and minimize their degradation under PEC conditions. This was to avoid leaching out of hazardous Cd2+ ions. Different parameters have been investigated for this purpose. Multi-deposition preparation, redox couple, and electrode etching affected electrode PEC characteristics. Consistent with earlier literature, annealing the electrode enhanced its conversion efficiency and stability. On the other hand, effect of cooling rate of pre-annealed CdS electrodes, prepared by CBD, on their PEC characteristics has been investigated here for the first time. Controlling the cooling rate was one major factor that affected CdS surface morphology, conversion efficiency and stability under PEC conditions. The major recommendation coming out here is that PEC characteristics of CdS thin film electrodes can be significantly enhanced by pre-annealing the electrode at ∼250 °C followed by its slow cooling.






