Diazepine
Synthesis of a New Series of Heterocyclic Scaffolds for Medicinal Purposes
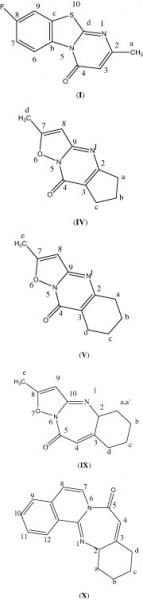
Mon, 2014-02-17 10:36 — Mohammed Saleem Ali-ShtayehA new series of substituted 8-fluro-4H-pyrimido[2,1-b] [1,3]benzothiazole-4-ones () substituted 7-methyl-4H-isoxazolo[2,3-a]pyrimidin-4-ones, and substituted 2-methyl-5,6,7,8-tetrahydro-9H-isoxazolo[2,3-a]pyridopyrimidin-9-ones, compounds I–VII, have been prepared via condensation of β-keto esters with 2-aminopyridine derivatives, in the presence of polyphosphoric acid. The same technique has also been used to prepare diazepine compounds, VIII–X, by condensation of a γ-keto ester with 2-aminopyridine derivatives. Details of synthetic procedures are shown. The new compounds have been characterized by elemental analysis, GC–MS, FT-IR and NMR spectrometry. Antibacterial, antifungal and anticancer (cytotoxic) activities, for three of these compounds, have been investigated and are presented.
Synthesis and Antibacterial Activity of Novel Curcumin Derivatives Containing Heterocyclic Moiety
Mon, 2013-05-13 18:22 — Adham S. Abu TahaA series of curcumin derivatives containing heterocyclic moiety have been synthesized and evaluated for their antibacterial activities. The chemical structures of the synthesized compounds were verified on the basis of spectral data and elemental analyses. Investigation of antimicrobial activity of the derivatives demonstrated the ability to inhibit Gram-positive microorganisms with zone of inhibition ranging from 14-18 mm, MIC ranging between 0.0625 and 0.25 mg/mL. Among all tested derivatives, diazepine 4 exhibited remarkable potency against Gram-positive bacteria S. aureus. An extensive study is underway to optimize the effectiveness of diazepine type of compounds and to determine their mode of action.
Synthesis of a New Saries of Hetrocyclic Scaffolds for Medicinal Purposes
Sun, 2013-04-07 12:51 — Ali BarakatA new series of substituted 8-fluro-4H-pyrimido[2,1-b] [1,3]benzothiazole-4-ones () substituted 7-methyl-4H-isoxazolo[2,3-a]pyrimidin-4-ones, and substituted 2-methyl-5,6,7,8-tetrahydro-9H-isoxazolo[2,3-a]pyridopyrimidin-9-ones, compounds I–VII, have been prepared via condensation of β-keto esters with 2-aminopyridine derivatives, in the presence of polyphosphoric acid. The same technique has also been used to prepare diazepine compounds, VIII–X, by condensation of a γ-keto ester with 2-aminopyridine derivatives. Details of synthetic procedures are shown. The new compounds have been characterized by elemental analysis, GC–MS, FT-IR and NMR spectrometry. Antibacterial, antifungal and anticancer (cytotoxic) activities, for three of these compounds, have been investigated and are presented.
Synthesis and Antibacterial Activity of Novel Curcumin Derivatives Containing Heterocyclic Moiety
Fri, 2013-02-15 22:08 — Othman A. HamedA series of curcumin derivatives containing heterocyclic moiety have been synthesized and evaluated for their antibacterial activities. The chemical structures of the synthesized compounds were verified on the basis of spectral data and elemental analyses. Investigation of antimicrobial activity of the derivatives demonstrated the ability to inhibit Gram-positive microorganisms with zone of inhibition ranging from 14-18 mm, MIC ranging between 0.0625 and 0.25 mg/mL. Among all tested derivatives, diazepine 4 exhibited remarkable potency against Gram-positive bacteria S. aureus. An extensive study is underway to optimize the effectiveness of diazepine type of compounds and to determine their mode of action.
Synthesis of a New Series of Heterocyclic Scaffolds for Medicinal Purposes
Wed, 2010-04-21 13:32 — Hikmat S. Hilal, D.Sc.A new series of substituted 8-fluro-4H-pyrimido[2,1-b] [1,3]benzothiazole-4-ones () substituted 7-methyl-4H-isoxazolo[2,3-a]pyrimidin-4-ones, and substituted 2-methyl-5,6,7,8-tetrahydro-9H-isoxazolo[2,3-a]pyridopyrimidin-9-ones, compounds I–VII, have been prepared via condensation of β-keto esters with 2-aminopyridine derivatives, in the presence of polyphosphoric acid. The same technique has also been used to prepare diazepine compounds, VIII–X, by condensation of a γ-keto ester with 2-aminopyridine derivatives. Details of synthetic procedures are shown. The new compounds have been characterized by elemental analysis, GC–MS, FT-IR and NMR spectrometry. Antibacterial, antifungal and anticancer (cytotoxic) activities, for three of these compounds, have been investigated and are presented.