Antifungal
Synthesis of Novel Biologically Active Mono Acid Esters Derived From The Constituents of Urtica Pilulifera
Sun, 2014-12-28 08:53 — Dr. Waheed J. JondiNew mono acid esters have been synthesized from the reaction of benzoic acid and mono-hydroxybenzoic acids with 2-phenoxyethanol separated from Urtica pilulifera, characterized, and screened for possible antioxidant, antifungal, antimicrobial and anticancer activities. 2-phenoxyethyl 4-hydroxy benzoate showed considerable activity against MCF-7 with IC50 is less than 62.5 µg/ml, and complete inhibition at a concentration less than 37.5 µg/ml against M. canis and less than 50 µg/ml against T. rubrum. On the other hand 2-phenoxyethyl 2-hydroxy benzoate reveals 70% of gentamicin against K. pneumoniae.
Exhaustive Extraction and Screening The Biological Activities of Heliotropium Hirsutissimum (Hairy Heliotrope): a Member of Palestinian Flora
Thu, 2014-12-11 15:03 — Nidal Amin JaradatObjective: Heliotropium hirsutissimum Grauer. is one of the
important folk plants that grow wildly in the mountains of Palestine and widely
used for several purposes by tribal peoples and traditional practitioners for
treatment of various skin inflammations as a paste, for that our study aimed to evaluate antibacterial and antifungal activities for this plant.
Methods: In our experiments, the entire plant exhaustively extracted by
n-hexane and ethanol mixed with an equal volume of triple distilled water to yield
2.36 g crude aqueous extract and 0.42 g crude organic extract from 25 g of the
plant and then screened for antifungal and antibacterial activities. Results: The plant aqueous extract showed antibacterial activities against all
Gram-positive bacteria with the greatest activity against Staphylococcus epidermidis,
its inhibition zone diameter (DIZ) was 16 mm equivalent to 50% of the DIZ of
imipenem a broad spectrum antibiotics. Furthermore, the plant aqueous extract activity against Bacillus subtilis and Staphylococcus
aureus were 21.7% of imipenem activity (10 mm) and 26% imipenem activity (12
mm), respectively. The minimum inhibition concentration (MIC) of the aqueous
extract was 30 mg/ml or less against all bacterial strains as well as against the fungi Candida albican. More specifically, the MIC ranged
between 2.7 mg/ml and 30 mg/ml with the lowest MIC value was 2.7 mg/ml against
S. epidermidis, while Pseudomonas aeruginosa, Escherichia coli, and C. albican
was all inhibited at the highest concentration of 30 mg/ml. There is no bacteriocidal or fungicidal effect of its extract at 30
mg/ml the highest concentration tested in our experiments. The organic extract
showed identical antibacterial activity of aqueous extract except in that it
had no activity against B. subtilis, as well as it had antibacterial activity against E. coli with DIZ of 50% of DIZ of imipenem (18 mm) and
antifungal activity against C. albican with DIZ 80% of DIZ of nystatin broad spectrum
antifungal compound (16 mm). Conclusion: The plant aqueous and organic extracts have antibacterial,
antifungal active compounds.
Synthesis of Novel Biologically Active Mono Acid Esters Derived From The Constituents of Urtica Pilulifera
Wed, 2014-02-19 13:17 — Mohammed Saleem Ali-ShtayehNew mono acid esters have been synthesized from the reaction of benzoic acid and mono-hydroxybenzoic acids with 2-phenoxyethanol separated from Urtica pilulifera, characterized, and screened for possible antioxidant, antifungal, antimicrobial and anticancer activities. 2-phenoxyethyl 4-hydroxy benzoate showed considerable activity against MCF-7 with IC50 is less than 62.5 µg/ml, and complete inhibition at a concentration less than 37.5 µg/ml against M. canis and less than 50 µg/ml against T. rubrum. On the other hand 2-phenoxyethyl 2-hydroxy benzoate reveals 70% of gentamicin against K. pneumoniae.
Synthesis of a New Series of Heterocyclic Scaffolds for Medicinal Purposes
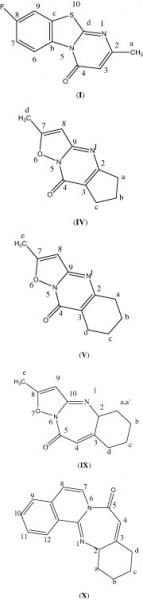
Mon, 2014-02-17 10:36 — Mohammed Saleem Ali-ShtayehA new series of substituted 8-fluro-4H-pyrimido[2,1-b] [1,3]benzothiazole-4-ones () substituted 7-methyl-4H-isoxazolo[2,3-a]pyrimidin-4-ones, and substituted 2-methyl-5,6,7,8-tetrahydro-9H-isoxazolo[2,3-a]pyridopyrimidin-9-ones, compounds I–VII, have been prepared via condensation of β-keto esters with 2-aminopyridine derivatives, in the presence of polyphosphoric acid. The same technique has also been used to prepare diazepine compounds, VIII–X, by condensation of a γ-keto ester with 2-aminopyridine derivatives. Details of synthetic procedures are shown. The new compounds have been characterized by elemental analysis, GC–MS, FT-IR and NMR spectrometry. Antibacterial, antifungal and anticancer (cytotoxic) activities, for three of these compounds, have been investigated and are presented.
Synthesis of a New Series of Heterocyclic Scaffolds for Medicinal Purposes
Wed, 2010-04-21 13:32 — Hikmat S. Hilal, D.Sc.A new series of substituted 8-fluro-4H-pyrimido[2,1-b] [1,3]benzothiazole-4-ones () substituted 7-methyl-4H-isoxazolo[2,3-a]pyrimidin-4-ones, and substituted 2-methyl-5,6,7,8-tetrahydro-9H-isoxazolo[2,3-a]pyridopyrimidin-9-ones, compounds I–VII, have been prepared via condensation of β-keto esters with 2-aminopyridine derivatives, in the presence of polyphosphoric acid. The same technique has also been used to prepare diazepine compounds, VIII–X, by condensation of a γ-keto ester with 2-aminopyridine derivatives. Details of synthetic procedures are shown. The new compounds have been characterized by elemental analysis, GC–MS, FT-IR and NMR spectrometry. Antibacterial, antifungal and anticancer (cytotoxic) activities, for three of these compounds, have been investigated and are presented.