Activated carbon
A Study of the Influence of Hydrophobicity of Activated Carbon on the Adsorption Equilibrium of Aromatics in Non-Aqueous Media
Tue, 2015-03-24 14:22 — Hassan ArafatOn the Adsorption of Aromatics on Oxygenated Activated Carbon in Nonaqueous Adsorption Media
Tue, 2015-03-24 14:06 — Hassan ArafatIn a previous study, H-bonding was postulated as a mechanism of adsorption for aromatics on oxygen-containing activated carbon. To verify this, the adsorption of phenol, aniline, benzene, and nitrobenzene was studied as a function of surface oxygen groups. It was determined that there is a linear correlation between total surface acidity and adsorption capacity for H-bonding adsorbates in cyclohexane. Flow microcalorimetry (FMC) and ultrasonic desorption tests also indicate stronger and less reversible adsorption bonds for H-bonding adsorbates. Reversibility
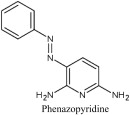
Pristine and Supported Zno-Based Catalysts for Phenazopyridine Degradation with Direct Solar Light
Mon, 2011-03-14 13:59 — Iyad SAADEDDINIn search for safe techniques to manage waste pharmaceutical compounds drained in water, solar-driven degradation of phenazopyridine (a model drug) was investigated in aqueous media using different ZnO-based catalyst systems. Naked ZnO, CdS-sensitized ZnO (ZnO/CdS) and activated carbon-supported ZnO (AC/ZnO) have been studied. Both naked ZnO and AC/ZnO were highly efficient in mineralizing phenazopyridine, reaching complete removal in ∼50 min, with AC/ZnO having the higher edge. The ZnO/CdS system showed lower efficiency, due to screening of light by CdS. Moreover, the tendency of CdS to leach out Cd2+ ions discouraged the use of CdS as sensitizer in this work. In both ZnO and AC/ZnO systems, the photo-degradation reaction was induced by the UV tail of the solar light. The visible region, with wavelength longer than 400 nm, failed to induce photo-degradation. The reaction was faster with higher catalyst loading, until a maximum efficiency was reached at a certain concentration. The rate of reaction increased with higher drug concentrations up to a certain limit. The effect of pH value was studied, and the catalysts showed highest efficiencies at pH close to 7. Stability of ZnO to degradation was studied. Both catalyst systems showed lowered efficiencies on recovery and reuse. The results suggest that complete mineralization of waste drugs, commonly dumped in sewage water, with direct solar light is a potentially feasible strategy using the AC/ZnO catalyst.
Pristine and Supported Zno-Based Catalysts for Phenazopyridine Degradation with Direct Solar Light
Mon, 2011-03-14 13:58 — Ahed Husni Abdel Razaq ZyoudIn search for safe techniques to manage waste pharmaceutical compounds drained in water, solar-driven degradation of phenazopyridine (a model drug) was investigated in aqueous media using different ZnO-based catalyst systems. Naked ZnO, CdS-sensitized ZnO (ZnO/CdS) and activated carbon-supported ZnO (AC/ZnO) have been studied. Both naked ZnO and AC/ZnO were highly efficient in mineralizing phenazopyridine, reaching complete removal in ∼50 min, with AC/ZnO having the higher edge. The ZnO/CdS system showed lower efficiency, due to screening of light by CdS. Moreover, the tendency of CdS to leach out Cd2+ ions discouraged the use of CdS as sensitizer in this work. In both ZnO and AC/ZnO systems, the photo-degradation reaction was induced by the UV tail of the solar light. The visible region, with wavelength longer than 400 nm, failed to induce photo-degradation. The reaction was faster with higher catalyst loading, until a maximum efficiency was reached at a certain concentration. The rate of reaction increased with higher drug concentrations up to a certain limit. The effect of pH value was studied, and the catalysts showed highest efficiencies at pH close to 7. Stability of ZnO to degradation was studied. Both catalyst systems showed lowered efficiencies on recovery and reuse. The results suggest that complete mineralization of waste drugs, commonly dumped in sewage water, with direct solar light is a potentially feasible strategy using the AC/ZnO catalyst.
Pristine and Supported Zno-Based Catalysts for Phenazopyridine Degradation with Direct Solar Light
Wed, 2010-04-21 13:32 — Hikmat S. Hilal, D.Sc.In search for safe techniques to manage waste pharmaceutical compounds drained in water, solar-driven degradation of phenazopyridine (a model drug) was investigated in aqueous media using different ZnO-based catalyst systems. Naked ZnO, CdS-sensitized ZnO (ZnO/CdS) and activated carbon-supported ZnO (AC/ZnO) have been studied. Both naked ZnO and AC/ZnO were highly efficient in mineralizing phenazopyridine, reaching complete removal in not, vert, similar50 min, with AC/ZnO having the higher edge. The ZnO/CdS system showed lower efficiency, due to screening of light by CdS. Moreover, the tendency of CdS to leach out Cd2+ ions discouraged the use of CdS as sensitizer in this work. In both ZnO and AC/ZnO systems, the photo-degradation reaction was induced by the UV tail of the solar light. The visible region, with wavelength longer than 400 nm, failed to induce photo-degradation. The reaction was faster with higher catalyst loading, until a maximum efficiency was reached at a certain concentration. The rate of reaction increased with higher drug concentrations up to a certain limit. The effect of pH value was studied, and the catalysts showed highest efficiencies at pH close to 7. Stability of ZnO to degradation was studied. Both catalyst systems showed lowered efficiencies on recovery and reuse. The results suggest that complete mineralization of waste drugs, commonly dumped in sewage water, with direct solar light is a potentially feasible strategy using the AC/ZnO catalyst.