Doped ceramics
Simultaneous Doping of Zn And Sb in Sno2 Ceramics: Enhancement of Electrical Conductivity
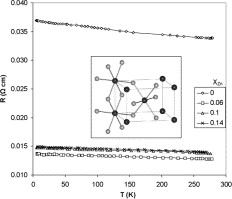
Mon, 2014-03-03 13:19 — Iyad SAADEDDINSnO2-based ceramics doped with Sb and/or Zn, have been prepared by solid state reaction at 1300 °C. The effect of dopants on electronic properties and sintering behavior has been studied. While undoped SnO2 pellets displayed very low electrical conductivities and lower densities, the Sb-doped ceramics showed higher electrical conductivity, with almost no densification and a significant antimony loss. On the contrary, a high densification and low conductivity are obtained for Zn-doped ceramics. Therefore, it is worthwhile to investigate SnO2 ceramics co-doped with Sb and Zn (SnO2:Sb:Zn) to combine the advantages of both dopants. X-ray photoelectron spectroscopy (XPS) analysis confirmed that Sb5+ is mainly substituted at the Sn4+ site for Sb-doped ceramics and is in agreement with Hall-measurements. In the case of SnO2 samples co-doped with Sb and Zn, XPS and Hall data confirmed the presence of both Sb5+ and Sb3++. The SnO2:Sb:Zn system exhibited enhanced electrical conductivity and high densities. In addition, the presence of Zn prevented the Sb evaporation during sintering.

Simultaneous Doping of Zn And Sb in Sno2 Ceramics: Enhancement of Electrical Conductivity
Wed, 2010-04-21 13:32 — Hikmat S. Hilal, D.Sc.SnO2-based ceramics doped with Sb and/or Zn, have been prepared by solid state reaction at 1300 °C. The effect of dopants on electronic properties and sintering behavior has been studied. While undoped SnO2 pellets displayed very low electrical conductivities and lower densities, the Sb-doped ceramics showed higher electrical conductivity, with almost no densification and a significant antimony loss. On the contrary, a high densification and low conductivity are obtained for Zn-doped ceramics. Therefore, it is worthwhile to investigate SnO2 ceramics co-doped with Sb and Zn (SnO2:Sb:Zn) to combine the advantages of both dopants. X-ray photoelectron spectroscopy (XPS) analysis confirmed that Sb5+ is mainly substituted at the Sn4+ site for Sb-doped ceramics and is in agreement with Hall-measurements. In the case of SnO2 samples co-doped with Sb and Zn, XPS and Hall data confirmed the presence of both Sb5+ and Sb3++. The SnO2:Sb:Zn system exhibited enhanced electrical conductivity and high densities. In addition, the presence of Zn prevented the Sb evaporation during sintering.