Electrochromic Devices Based on in Situ Polymerised EDOTand Prussian Blue: Influence of Transparent Conducting Oxide and Electrolyte Composition—Towards Up-Scaling
Tue, 2014-03-04 11:29 — Iyad SAADEDDIN
Journal Title, Volume, Page:
New J. Chem.
Year of Publication:
2011
Preferred Abstract (Original):
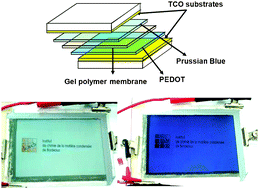
Inorganic/organic (hybrid) complementary electrochromic devices (ECDs) of the type [transparent conducting oxide (TCO)//inorganic counter electrode/hydrophobic electrolytic membrane/polymeric working electrode//TCO] were assembled. The working electrodes consisted of spin-coated polymer films prepared by moderator-controlled in situ oxidative chemical polymerisation of 3,4-ethylene dioxythiophene (EDOT). Thin, galvanostatically deposited Prussian Blue (PB) films were employed as counter electrodes. Besides F :
: SnO2(FTO)/glass and Sn
SnO2(FTO)/glass and Sn :
: In2O3 (ITO)/glass, a flexible ITO/PET film was alternatively used for materials deposition. In order to attain the maximum device performance, the PB charge capacity was monitored and adapted to the capacity of the EDOT polymer films. The two electrochromic electrodes were separated by a novel hydrophobic polymer electrolyte based on a gel of 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (BMI-TFSI) and poly(methylmethacrylate) (PMMA), with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as the salt. The influence of two parameters—ITO sheet resistance and the PMMA content in the electrolyte—on the final device properties was investigated. The ITO sheet resistance value proved to be crucial for the switching kinetics. The variation of the weight ratio of PMMA in the electrolyte showed that the effect on the kinetics is small whereas the change in absorbance is highly affected. The properties of the complementary glass-based devices were eventually compared to the corresponding plastic-based electrochromic elements. First attempts to scale up the technology were made for flexible 12 × 15 cm2 (active area) devices.
In2O3 (ITO)/glass, a flexible ITO/PET film was alternatively used for materials deposition. In order to attain the maximum device performance, the PB charge capacity was monitored and adapted to the capacity of the EDOT polymer films. The two electrochromic electrodes were separated by a novel hydrophobic polymer electrolyte based on a gel of 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (BMI-TFSI) and poly(methylmethacrylate) (PMMA), with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as the salt. The influence of two parameters—ITO sheet resistance and the PMMA content in the electrolyte—on the final device properties was investigated. The ITO sheet resistance value proved to be crucial for the switching kinetics. The variation of the weight ratio of PMMA in the electrolyte showed that the effect on the kinetics is small whereas the change in absorbance is highly affected. The properties of the complementary glass-based devices were eventually compared to the corresponding plastic-based electrochromic elements. First attempts to scale up the technology were made for flexible 12 × 15 cm2 (active area) devices.